In preclinical research, scientists test their ideas in a laboratory or in animals for biomedical prevention strategies. Whereas clinical research refers to studies in animals, But in preclinical research encompasses everything that happens before a candidate is called for human testing. In preclinical testing, it answers a few ranges of questions. First of all, scientists insist on their ideas for new projects and they take forward if their ideas have a positive sign.
Vitro and Vivo experiments in preclinical research
The scientist acquires a new strategy in experimental microbicide. The microbicide is combined with HIV in a laboratory to check whether it stops the HIV activity. Vitro experiments are done in lab dishes or test tubes but not in animals and the vivo experiments take place in animals and people. If the experimental strategy works in vitro, then the scientist moves with animals and humans. The experimental candidate’s safety, toxicity and dosing strategy, as well as HIV risk reduction benefit information, should be known. Mice, guinea pigs, rabbits and monkeys are some of the animals used in preclinical vivo experiments.
Microbicide and Immune studies
The preclinical research depends on the strategy the scientists have taken for the testing process. At the time of the experiment; scientist made vaccine studies on animal blood to know the immune response. Microbicide studies help the scientists to look for any irritation in the vaginal linings or the rectal of the animals. Identify the candidate can reduce the risk of HIV infection, by conducting a preliminary test on non-pirates creatures.
Experimental strategy in animals
The animals used for preclinical research are exposed to or challenges with the strains of SHIV or SIV. These two are HIV like viruses that can cause disease in the monkey. The animals which receive experimental strategy will face different responses at the time of testing. If the animals have a lower defect in the experimental strategy then the preliminary test evidence of some risk reduction benefit. By using animals studies couldn’t predict whether they protect human and non-human primates because they have a different immune system and vary response in HIV or HIV like viruses. Human research is important and the process is so uncertain.
FDA requirements
FDA requires scientists to use good practice laboratories. For preclinical laboratory research, it requires medical product developments regulation. The regulations posses’ minimum requirements such as facilities, writing protocols, study reports and operating producers required for the safety of the FDA regulated product. The preclinical research is not very large, these studies provide information on toxicity and dosing levels. After the testing, the scientists review their findings and decide whether the FDA drug should be tested in people
TREAT-NMD fragmentation process
TREAT-NMD requires in reducing fragmentation of harmonizing tools used in preclinical research. The goal of the research is to use a limited number of animal models and experimental protocols should be inappropriately used by research groups all over the world. By improving the comparability of the clinical work and accelerate the inventions of new work.
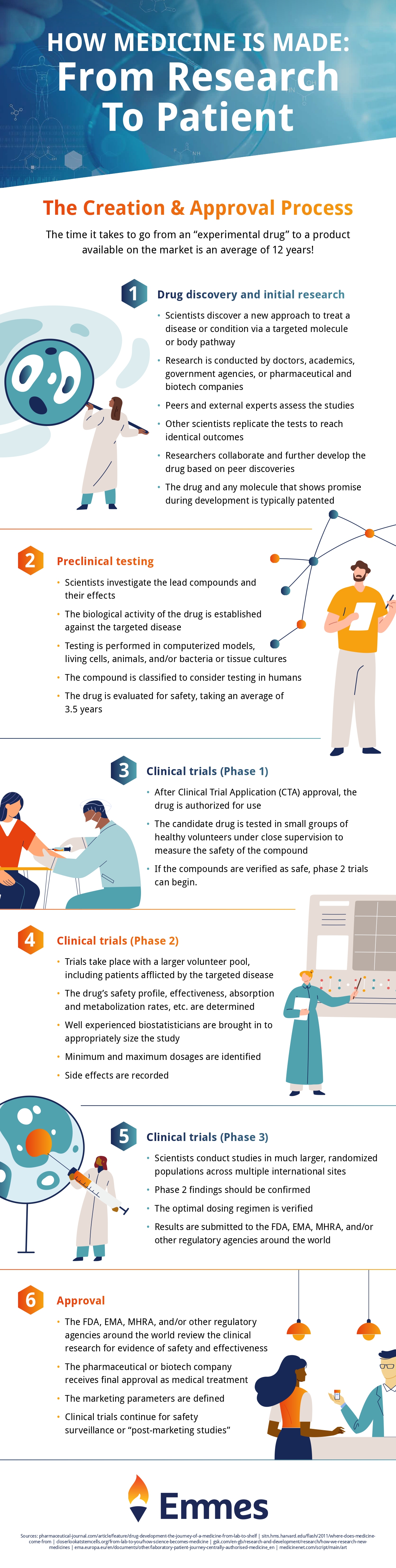
Pre-clinical research is just one stage in the medicine making process. Check out the infographic below to see how your medicine is made, from start to finish!
Infographic provided by The Emmes Company, a clinical data management organization